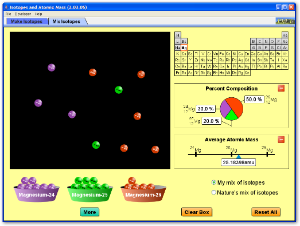
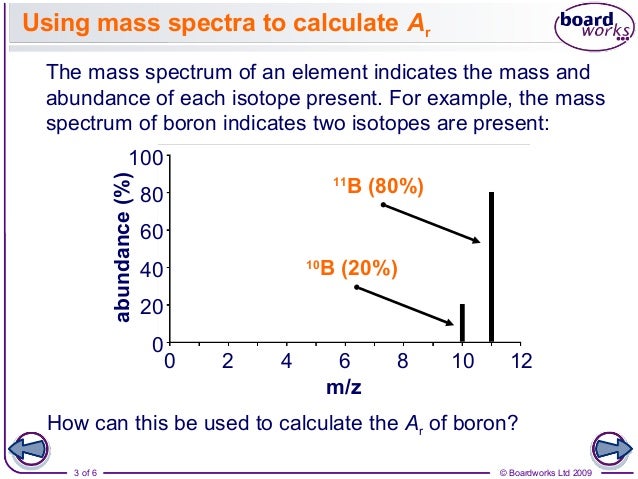
The relative atomic mass of Gallium is 69.7. Natural gallium consists of two isotopes. The isotope Ga (mass-69 and atomic number-31) is 60.5% abundant and its relative isotopic mass is 69.0. The relative atomic mass is the weighted average of the isotopic masses. Example: Chlorine has two isotopes 35 Cl and 37 Cl, with relative abundance of 75% and 25% respectively. This means that in any naturally occurring sample of chlorine 75% of the atoms are Cl-35 atoms and 25% of the atoms are chlorine-37 atoms. Relative mass (c) explanation of the terms: relative isotopic mass (mass compared with 1/12th mass of Carbon-12). Relative atomic mass, A r, (weighted mean mass compared with 1/12th mass of Carbon-12),. Based on the mass of a 12 C atom, the standard for atomic masses. Indicate that it is not present in nature or that a meaningful natural abundance cannot be given. The isotopic mass data is from G. 1993, 565, 1-65 and G. 1995, 595, 409-480. The percent natural abundance data is from the 1997 report of the IUPAC Subcommittee for Isotopic.
Version History and References | Disclaimer
Developers and Contributors:
Relative isotopic mass is known to be the relationship between the mass of an isotope of an element relative to the mass of an isotope of the atom. See full answer below. Become a member.
J. S. Coursey, D. J. Schwab, J. J. Tsai, and R. A. Dragoset
NIST Physical Measurement Laboratory
The atomic weights are available for elements 1 through 118 and isotopic compositions or abundances are given when appropriate. The atomic weights data were published by J. Meija et al in Atomic Weights of the Elements 2013, and the isotopic compositions data were published by M. Berglund and M.E. Wieser in Isotopic Compositions of the Elements 2009. The relative atomic masses of the isotopes data were published by M. Wang, G. Audi, A.H. Wapstra, F.G. Kondev, M. MacCormick, X. Xu1, and B. Pfeiffer in The AME2012 Atomic Mass Evaluation.
Atomic Mass With Isotopes
These data have been compiled from the above sources for the user's convenience and does not represent a critical evaluation by the NIST Physical Measurement Laboratory.
Related Links
Relative Isotopic Mass Meaning
Development of this database was funded in part by NIST's Systems Integration for Manufacturing Applications (SIMA) Program.
Online: September 1999 | Last update: January 2015

